
SFRN Center on Cardiovascular Kidney Metabolic Syndrome: Heterogeneity in Women
Purpose
This Request for Proposals seeks applications that can address critical questions and stimulate significant advances in our understanding of Cardiovascular Kidney Metabolic Syndrome in women. Whereas studies should be geared toward clarifying mechanisms present in women, studies that include men/males are also appropriate as needed for comparative purposes.
Background
CARDIOVASCULAR DISEASE IN WOMEN
Cardiovascular disease (CVD) is the number 1 killer of women, responsible for 1 in 4 deaths each year. For decades there has been an assumption that women are protected against CVD, presumably due to their hormonal profile. In part due to this false assumption, there is a relative paucity of data addressing CVD specific to women. And whereas the death rate due to heart disease does remain higher in men than women1, there are a number of areas in which CVD-related risks are higher or distinct in women compared to men. As one example, it is now clear that the clinical presentation of symptoms during a myocardial infarction (MI) differs between the sexes. This may lead to a delay in care and, therefore, worse post-MI outcomes for women. Additionally, there exists race- and ethnic-based evidence to suggest that women from underrepresented races and ethnicities suffer even poorer outcomes compared to their white counterparts after an MI.2
To better understand CVD in women, a classification system has been devised to organize the possible causes of CVD into common risk factors (i.e., present in women and men) and women-specific risk factors.3 The latter are health conditions unique to or more prominent in women that can exacerbate CVD risk.
Common risk factors: Diabetes is a major cause of CVD and chronic kidney disease (CKD), among other health conditions. In general, individuals with diabetes have twice the risk of developing CVD compared to those who do not have diabetes, and the risk of CVD in those with diabetes is significantly higher in women than men.4 Smoking is also associated with a higher CVD risk in women than men.3 With regard to lipids, the menopausal transition leads to an increase in LDLs (which facilitate CVD) such that older women have higher LDLs relative to men later in life.3
Women-specific risk factors: Endocrine-related conditions such as Polycystic Ovary Syndrome (PCOS) and menopause alter a woman’s hormonal profile, resulting in a less favorable state for the cardiovascular system. PCOS is often linked with insulin resistance, which can increase the risk of developing diabetes in affected women. The age of menopause marks differences in CVD risk. For instance, early menopause (before the age of 40 yrs), especially when the result of ovariectomy, results in a considerable increase in CVD Relative Risk in comparison to the risk observed in those experiencing menopause near the age of 50.3
Pregnancy can increase CVD-related risks in a number of ways. Pregnancy-related complications such as preeclampsia (hypertension and proteinuria after 20 weeks of gestation), pregnancy-induced hypertension, or gestational diabetes impact roughly 3-20% of pregnancies.5 Both hypertensive conditions increase the risk for the development of hypertension, heart failure and/or diabetes later in life.5,6 Gestational diabetes occurs during pregnancy when insulin resistance develops; this increases by 8-fold the likelihood of developing type 2 diabetes later in life.7
Women are also more likely to have auto-immune diseases such as lupus and rheumatoid arthritis. These systemic inflammatory diseases are both risk factors for CVD.8 Inflammation caused by tumor necrosis factor-α, interleukin-6, and other factors promotes endothelial dysfunction, vessel stiffness, premature atherosclerosis, and coronary microvascular dysfunction.9
Women continue to be underrepresented in clinical cardiovascular trials, leaving a gap in our understanding of best treatment practices. Women from underrepresented races and ethnicities and women from lower socio-economic status groups are particularly underrepresented in clinical trials. Taken together, the differences in CVD risk between men and women and the relative lack of studies focused on cardiovascular health in women clearly warrant further investigation. One condition in particular for which little is known with respect to women is Cardiovascular Kidney Metabolic (CKM) syndrome.
CARDIOVASCULAR KIDNEY METABOLIC SYNDROME
Individuals with elevated metabolic risk factors have increased risk for both CVD and chronic kidney disease (CKD). A relationship between heart disease and kidney disease (and vice versa) is also understood. In recent decades, the understanding that metabolic disease, CVD and CKD exists concurrently in individuals has become more common, leading to poor health outcomes. Cardiovascular Kidney Metabolic Syndrome has recently been defined by the AHA and provides a framework for understanding disease progression and outcomes in individuals experiencing these conditions10,11 (See Figure 1).
Ndumele et al.10 defined a 4-stage model for the progression of CKM pathophysiology (See Figure 2). According to a recent analysis of the National Health and Nutrition Examination Survey (NHANES) data, nearly 90% of adults in the U.S. meet the criteria for ≥ stage 1 CKM syndrome,12 and one in three adults have at least three of the risk factors for CKM.13
HETEROGENEITY OF CKM SYNDROME IN WOMEN
CKM syndrome is characterized by various risk factors and stages, leading to considerable heterogeneity. Many risk factors for cardiovascular disease (CVD) in women - noted above - also increase the risk of CKM syndrome.10 These include early menopause, pregnancy complications, polycystic ovarian disease, autoimmune diseases, anxiety, and depression.10 Furthermore, factors such as diabetes, cardiovascular disease, and chronic kidney disease (CKD) are known to affect women differently than men. For example, studies have shown an increased progression of diabetes-related risk factors in women,14 a higher prevalence of cardiovascular disease in women with diabetes compared to men with diabetes,4 and a roughly 20% higher prevalence of CKD in women compared to men. It is important to recognize that while changes in hormone levels may impact some of these conditions, other factors play significant roles as well.
Additional aspects of heterogeneity include variability of disease within weight categories, and variability in the rate at which individuals progress through the stages of CKM stage.11 These heterogeneities, including the extent to which variability between women and men may exist, are not well understood.
GAPS IN RESEARCH
As documented above, considerable heterogeneity has been described for CKM syndrome, including many risk factors and conditions that are exclusively or preferentially associated with women. In a large proportion of these conditions, however, the underlying mechanisms and/or opportunities for intervention are not understood. An understanding of these conditions is critically needed to more effectively impact the course of CKM syndrome and foster the development of targeted preventive strategies, tailored therapeutic approaches and ultimately improved outcomes in those with CKM syndrome. Because social drivers of health play a critical role in development and progression of CKM syndrome, use of a socioecological framework to consider societal factors and the roles of community, relationships and individual behaviors could provide important insight. At the biological level, understanding of the interaction of hormonal, metabolic and inflammatory pathways, as well as genetic, epigenetic and microbiome considerations may provide important fundamental insight into CKM syndrome.
Network Overview and Structure
This SFRN on Cardiovascular Kidney Metabolic Syndrome: Heterogeneity in Women will consist of at least three Centers, each of which will propose novel research studies to address this issue. Funded Centers are expected to collaborate on solving the core issues underlying this problem, including via development of a common network-wide collaborative project (see below).
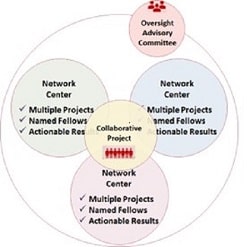 NETWORK CENTERS – Each Center application will include two or three research projects. Applicants may choose the scientific approach (basic, clinical/translational, or population health studies) that most appropriately addresses the research question(s) being posed in each project. Projects should be individually meritorious and complement the broader theme of the center.
NETWORK CENTERS – Each Center application will include two or three research projects. Applicants may choose the scientific approach (basic, clinical/translational, or population health studies) that most appropriately addresses the research question(s) being posed in each project. Projects should be individually meritorious and complement the broader theme of the center.
Projects may be from a single institution or from multiple institutions. A project principal investigator (PI) will lead each research project, and must have the necessary research team, required infrastructure and ability to conduct the proposed research.
One overall Center Director must be named (Co-Center Directors are not permitted). This key person will facilitate activities within their Center and work closely with the other Network Center Directors to coordinate activities across the Network, including end-of-network deliverables.
OVERSIGHT ADVISORY COMMITTEE – An Oversight Advisory Committee (OAC) will be established to facilitate the success of this SFRN. The OAC will be comprised of volunteers who are subject matter experts in the focus areas.
Representative Approaches Responsive to this RFP and Study Population(s)
REPRESENTATIVE APPROACHES RESPONSIVE TO THIS RFP
The intent of this initiative is to support a collaborative network of researchers whose collective efforts will lead to enhanced understanding of the heterogeneity of CKM syndrome in women. All proposed projects must address the heterogeneity of CKM syndrome in women. Within that context and as presented above, an array of potential areas of investigation exists. Both human studies and appropriately designed animal models that can foster understanding of heterogeneity in women may be proposed. Potential areas of investigation are noted below; this list is not exhaustive and is not meant to direct applicants to a particular area of study.
- Mechanisms of CVD development in CKM: More research is needed to understand heterogeneity of development of CVD conditions in distinct populations (e.g., sex, varying race and/or ethnicity, age, or co-morbidities). Some specific areas of assessment might include analysis of genetic and epigenetic traits, biomarker and/or molecular signaling profiles with CKM stage transition and determinants of progression from subclinical to clinical CVD.
- Mechanisms of risk factor heterogeneity in overweight individuals: Extensive heterogeneity exists in women at various weight categories, including across racial and ethnic groups. Exploration of mechanisms underlying this heterogeneity would increase our fundamental understanding of CKM.
- The impact of Social Determinants of Health in CKM heterogeneity: SDOH are well known to influence a large array of parameters of health. As CKM has only recently been conceptualized, there is a clear need for better understanding the influence of biological predisposition and of SDOH on CKM risk in underrepresented female populations.
- The role of environment and community in heterogeneity and progression of CKM: The impact of environment and community on several indices of health has been uncovered in recent years. The extent to which these factors impact the progression of CKM is not understood.
- Novel therapeutic treatment strategies: The development in recent years of novel therapeutics such as SGLT2 inhibitors and GLP-1 receptor agonists provide an opportunity to explore novel prescribing approaches. Optimal strategies for use in those with varying risk factors for and at different stages of CKM syndrome are needed to improve outcomes.
STUDY POPULATION(S)
- The research question(s) being pursued must have as a focus understanding of that particular question in women (or females in animal studies). Studies in men/males are also acceptable as comparison groups.
- For studies involving human subjects, projects must include study participants who are underrepresented. The proportion of these individuals in proposed studies should be reflective, at a minimum, of their representation in the local/regional population from which subjects will be recruited.
- It will be important for applicants to design studies that incorporate both realistic recruitment goals and sufficient statistical power to ensure valid results.
Application Details
NETWORK CENTER APPLICATION DETAILS
Award Duration: Four (4) years
Number of Awards: The AHA anticipates awarding at least three (3) Network Center grants to establish this SFRN. Awardees will be selected based on scientific merit and how each group aligns with AHA’s mission and goals.
Collaborative Project: During Year 1 of the Network, the Centers will be required to develop a Network-wide Collaborative project, with cooperation from the Network Oversight Advisory Committee (OAC). The Collaborative project will start in Year 2. The AHA has set aside money for this effort, not to exceed $1,800,000 for the Network. More details on the Collaborative project will be made available after the Centers are named.
Award Amount: The maximum budget amount a Center applicant may request is $4,400,000. The AHA reserves the right to determine the final award amount for competitive projects based on need and potential impact.
Appropriate Budget Items:
- Salary and fringe benefits for the Center Director, Principal Investigators, three named fellows, collaborating investigator(s), and other participating research staff or faculty.
- Project-related expenses, such as salaries of technical personnel essential to the conduct of the project, supplies, equipment, travel, and publication costs in accordance with institutional and AHA policies.
- Centers should use award dollars to pay for travel to two required face-to-face (as feasible), network-wide meetings each year and other meetings where SFRN research is presented.
- Additional details on bi-annual meetings will be conveyed to awarded centers following award activation. Centers should anticipate hosting at least one of the meetings on a rotating basis. The purpose of both meetings is to share results across the network and identify and act on potential collaborative opportunities. Additionally, there will be virtual meetings if face-to-face travel is not available. More information will be provided upon award and once travel options become clear.
- Institutional indirect costs for operating expenses may be charged up to ten percent (10%) of the total expenditures each year on awards at the awardee institution. Any subcontract awardee institution (if applicable) is allowed institutional indirect costs up to ten percent (10%) of the total expenditures of the subcontract. The awardee institution may not charge indirect costs on the direct costs of a subcontract.
| Sample Center Budget | Center Totals |
|---|---|
| Projects TWO or THREE projects over four years. Maximum of $3.23M to be divided between/among the projects It is not required to spend funds equally across projects or years. | $3.23 M |
| Fellows Each center must train 3 postdoctoral fellows over the four-year grant period (for example, one fellow in years 1-2; one fellow in years 2-3; one fellow in years 3-4). Fellows must maintain a minimum of 75% effort to research training. See additional requirements for fellow appointment in the Named Fellows section of the RFP. Up to $75,000 per fellow each year (salary +benefits) | $450 K |
| Center Leadership The ONE Center Director (CD) must commit at least 20% effort. A maximum of $50,000 salary (salary includes fringe/benefits) per year to cover effort associated with directing the Center. | $250 K |
| Center Travel Costs Covers travel for Center personnel to attend network meetings and other integration activities. $10,000 per year must be allocated to Center Travel. | $40 K |
| One-time hosting of face-to-face scientific meeting | $30 K |
| Direct Costs (Total) Research Dollars | $4.00 M |
| Indirect Costs AHA Policy allows for a maximum of 10% for indirect costs | $400 K |
| Total | $4.4 M |
Note for Center Applicants: Each Center may have one Center Director. This person will be responsible for the progress of the projects and overseeing the total budget for their grant. If awarded, the principal investigators and the institution assume an obligation to expend grant funds for the research purposes set forth in the application and in accordance with all regulations and policies governing AHA grant programs.
Center Directors and Project Principal Investigators:
- Must possess an MD, PhD, DO, DVM or equivalent doctoral degree at time of application.
- Must have a faculty or staff appointment.
- May hold another AHA award simultaneously.
- Must demonstrate a 20% minimum effort requirement for the Director and a 10% minimum effort requirement for Principal Investigators (PI) of Center projects.
- These responsibilities are mutually exclusive, i.e., a Center Director who also serves as a Project PI must contribute a combined effort of 30%. Each named Director and PI must be able to commit the minimum effort required and may not split these efforts across more than one person.
Directors must have one of the following designations:
- U.S. citizen
- Permanent Resident
- Pending Permanent Resident (must have applied for permanent residency and have filed Form I-485 with the U.S. Citizenship and Immigration Services and have received authorization to legally remain in the U.S., having filed an Application for Employment Form I-765)
- G-4 Visa – family member of employee of international organizations and NATO
Principal Investigators of proposed projects must have one of the following designations:
- U.S. citizen
- Permanent Resident
- Pending Permanent Resident (must have applied for permanent residency and have filed Form I-485 with the U.S. Citizenship and Immigration Services and have received authorization to legally remain in the U.S., having filed an Application for Employment Form I-765)
- E-3 Visa – specialty occupation worker
- H1-B Visa – temporary worker in a specialty occupation
- O-1 Visa – temporary worker with extraordinary abilities in the sciences
- TN Visa – NAFTA professional
- G-4 Visa - family member of employee of international organizations and NATO
Named Fellows
The AHA’s aim is to help end historical structures and workplace cultures that advertently or inadvertently treat people inequitably based on race, ethnicity, gender, sexual orientation, age, ability, veteran status or other factors. Therefore, AHA strongly recommends at least half of the named fellows belong to a group that is under-represented in science. The AHA believes diversity and inclusion is an essential component to driving its mission and strongly encourages applications by women, underrepresented racial and ethnic groups in the sciences, military veterans, people with physical and mental impairments, individuals from disadvantaged backgrounds, members of the LGBTQ+ community, and those who have experienced varied and non-traditional career trajectories.
Each fellow must have one of the following designations:
- U.S. citizen
- Permanent Resident
- Pending Permanent Resident (must have applied for permanent residency and have filed Form I-485 with the U.S. Citizenship and Immigration Services and have received authorization to legally remain in the U.S., having filed an Application for Employment Form I-765)
- E-3 Visa – specialty occupation worker
- H1-B Visa – temporary worker in a specialty occupation
- O-1 Visa – temporary worker with extraordinary abilities in the sciences
- TN Visa – NAFTA professional
- J-1 Visa – exchange visitor
- F-1 Visa – student
- G-4 Visa - family member of employee of international organizations and NATO
A named fellow may not hold another comparable fellowship award, although the institution may provide supplemental funding. Fellows may not hold a faculty or staff appointment, except for MD or MD/PhD trainees who also maintain clinical responsibilities. These fellows may hold the title of instructor or similar due to their patient care responsibilities but must devote at least 75% effort to research training.
*All awardees must meet the citizenship criteria throughout the duration of the award.
Peer Review
GENERAL: Peer Review will be a two-phase process. Projects/Science from the Network Centers will be scored during Phase 1. Network Center applications that advance past Phase 1 will undergo a separate Phase 2 review that will focus on the overall vision of the center, synergy and collaborative possibilities within a Center (via the Center application) and across Centers, and the training plan and environment. Phase 2 will occur 2-4 weeks after Phase 1 review. Criteria for both phases of review follow.
Peer Review Criteria for PROJECT Applications
Phase 1 Review
Each PROJECT within a Center application will be scored individually according to the criteria below.
Projects – Potential impact of the project on research in the field of the designated research topic; strengths of applicant investigators (qualifications, expertise and productivity); potential for collaboration or synergy of projects; scientific content; background; preliminary studies; detailed specific aims; approach detail; analytical plan; sample size; data management; significance; innovation; individual project scientific merit; and total project coordination (within and among projects). Projects will be rated on the following areas:
- Approach: Are the conceptual framework, design, methods and analyses adequately developed, well-integrated, well-reasoned and feasible (as determined by preliminary data) and appropriate to the aims of the project? Does the applicant acknowledge potential problem areas and consider alternative tactics? Does each applicant develop a plan for interoperability of data between Centers and with National or International Standards?
NOTE: Applicants must explain how relevant biological variables, such as sex, are factored into the research design, analysis and reporting. Furthermore, very strong justification from the scientific literature must be provided for applications proposing to study only one sex. - Innovation: Is the project original and innovative? For example: Does the project challenge existing paradigms and address an innovative hypothesis or critical barrier to progress in the field? Does the project develop or employ novel concepts, approaches, methodologies, tools or technologies for this area?
- Investigator(s): Is the investigator(s) appropriately trained and well-suited to carry out this work? Is the work proposed appropriate to the experience level of the principal investigator and other researchers? Does the investigative team bring complementary and integrated expertise to the project (if applicable)? Project PIs must dedicate at least 10% to the project.
- Significance: Does this study address an important problem related to cardiovascular kidney metabolic syndrome, specifically exploring the heterogeneity of disease in women? If the aims of the application are achieved, how will mechanistic understanding of mediators related to CKM syndrome be advanced? What will be the effect of these studies on the concepts, methods and technologies that drive this field?
- Environment: Does the scientific environment in which the work will be done contribute to the probability of success? Do the proposed studies benefit from unique features of the scientific environment, or subject populations, or employ useful collaborative arrangements? Is there evidence of institutional support?
- Impact: How does the project relate to and support the mission of the AHA – To be a relentless force for a world of longer, healthier lives?
- Synergy: How does this project enhance the Center and the additional science project(s)? i.e., does this project enhance the likelihood that the collective Center outcomes will exceed outcomes of the individual sum of its distinct components? Synergy is the ability of a group to produce something greater than the sum of its parts; the ability of the group to outperform even its best individual member. Only projects that demonstrate synergy will move forward to Phase 2.
- Lay Summary/Summary for Non-Scientists: How well written is the lay summary in explaining to a non-scientist audience the research proposed and importance? Does the Lay Summary adequately explain the major health problem being addressed by this study? Does it provide specific questions and how the projects will address them? Does it provide information on the overall impact of this work and the potential advances in the field? Does it relay how the proposal supports the mission of the AHA?
Peer Review Criteria for CENTER Applications
Phase 2 Review
Each NETWORK CENTER moving beyond Phase I Review will be scored on the following:
- Synergy – A clear vision of scientific direction is expected. A Center should be viewed as a group of interrelated research projects, each of which is not only individually scientifically meritorious, but also complements the other projects and contributes to an integrating theme. Describe the rationale for the total program. Explain the strategy of achieving the objectives of the overall program and how each individual project relates to the strategy. Describe the synergies and interactions among projects and their investigators. Is there evidence of synergy among the projects and training component of the Center?
- Collaboration – History of collaboration, as well as the ability and commitment to collaborate with other institutions, investigators and within the applicant institution as well as within the awarded Network. Defined and detailed process for collaboration with other sites in addition to within and among the proposed projects; plans to actively participate in a collaborative network. Evidence of formal training in leadership skills with an emphasis on collaborative leadership will be favorably reviewed. What collaborations do you envision between investigators working on individual projects?
- Interaction Plan within and among this Network and other AHA Networks (if appropriate) – Plan for and commitment to sharing knowledge and methods, providing a stimulating atmosphere for research collaborations, and providing networking opportunities for trainees. Cited strategies for communication and interaction among the Centers. Centers proposing clinical projects must document that they have sufficient volume of patients from all identified study populations to ensure robust results are achievable.
- Training component – A detailed plan for developing and implementing a postdoctoral training program that includes clinical (M.D., D.O., PharmD) or Ph.D. training in research in the field outlined by the RFA; qualifications and characteristics of current and anticipated trainees; didactic and practicum training opportunities; plan for the selection of prospective fellows and how funded fellows’ ongoing progress will be guided via an individual development plan (IDP) and evaluated at least annually. Plan for involving fellows in annual Center meetings and Center-to-Center visits, along with identifying opportunities for fellows to work with established investigators at other network Centers; ability to track trainees; conferences and meeting participation for trainees; documentation of a ready supply of fellows; and history of successful fellowship training for researchers in the appropriate research topic.
- Center Director – Qualifications of the Director to provide scientific and administrative leadership for the Center; demonstrated ability to lead others, along with experience and commitment to the success of the Center, the projects contained within, and the Network. Documented evidence of willingness to collaborate with others outside their institution to share ideas, science, etc., to advance the research in the intended area.
- Investigator Team – Qualifications of each PI to provide scientific and administrative leadership for their respective projects; demonstrated commitment of each PI, and experience in the area(s) of studies proposed; qualifications of investigators, and co-investigators and the research team; training experience.
- Diversity of the Research Team – In keeping with AHA’s core values of diversity and inclusivity, AHA is committed to broadening the diversity of investigators supported by programmatic, multi-investigator initiatives it offers. As such, the AHA strongly recommends that at least 25% of key personnel of the research team belong to groups who are under-represented in science and medicine. The AHA believes diversity and inclusion is an essential component to driving its mission and strongly encourages applications by women, underrepresented racial and ethnic groups in the sciences, military veterans, people with physical and mental impairments, individuals from disadvantaged backgrounds, members of the LGBTQ+ community, and those who have experienced varied and non-traditional career trajectories. Applicants should address the diverse composition of the proposed research team and should comment on steps their institution(s) has taken/is taking to expand and support diverse investigators.
- Environment – Institutional commitment, resources and facilities to sustain the Center; institutional resources available to complete the project; analytical resources available to the project; letter from Center Director’s Department Head assuring the department and institution’s support of the Center along with confirmation that the Center Director will devote at least 20% effort towards the Center. Other Center personnel may be appointed to assist the Director in the administration of the Center. However, the Director will be required to devote 20% effort to the Center.
For more information on Peer Review of submitted applications, including information on reverse site visits, see the Peer Review section of the SFRN General Information page on the AHA SFRN website.
Applicants are prohibited from contacting AHA peer reviewers. This is a form of scientific misconduct and will result in removal of the application from funding consideration and institutional notification of misconduct.
AWARD SELECTION - Final funding decisions are subject to approval by the AHA.
Relevant Policies and Requirements
Institutional Eligibility / Location of Work:
AHA awards are limited to U.S.-based non-profit institutions, including medical, osteopathic and dental schools, veterinary schools, schools of public health, pharmacy schools, nursing schools, universities and colleges, public and voluntary hospitals and others that can demonstrate the ability to conduct the proposed research. Applications will not be accepted for work with funding to be administered through any federal institution or work to be performed by a federal employee, except for Veterans Administrations employees.
The Centers are not transferable to other institutions. An institution may submit only one Center (and related Projects) application in response to this RFP. Individuals at the applicant institution who are not participating in their institution’s center and project(s) application may participate in a separate institution’s Center application. Individuals other than the Center Director who are participating in their institution’s Center application, may participate in a separate institution’s Center application. The application may include individuals and/or projects at more than one institution provided there is evidence supporting the likelihood of a successful interaction among research and training personnel.
It is the responsibility of the submitting institution to ensure that only one proposal is submitted for the institution or to coordinate across several institutions to create a single application. The Center Director’s institution will maintain fiscal responsibility for the entire award.
Use of AHA’s Precision Medicine Platform: Applicants are encouraged to make use of AHA’s Precision Medicine Platform (PMP), powered by Amazon Web Services.
- The PMP supports cloud computing in a secure and private workspace and enables investigators to collaborate and analyze data securely. Each Project will receive Amazon Web Services computational credits to offset the cost of using the platform.
- Data analysis is enabled in secure workspaces by a friendly web user interface that allows researchers to code in various languages, including R and Python and use statistical software including but not limited to SAS and R studio. The most up-to-date machine learning and artificial intelligence software available from Amazon Web Services is also included. For a full list of the analytical tools available, please see precision.heart.org/workspace/about. Researchers are also able to upload their own tools.
- To learn more about the Precision Medicine Platform and how it can enable your research, please access the following videos. The first (Learn more about the platform – video 1) provides a high-level overview, while the second (Explore the capabilities of the platform – video 2) provides more detail about accessing data and analytical tools, data storage, and sharing of data. Additional questions can be answered on the AHA Application Resources page under the Precision Medicine Platform Header.
- The PMP is HIPAA and FedRAMP compliant.
Interim Assessment: Awardees must report progress on a minimum annual (once per year) basis. Progress may take the form of a required written report in addition to video conferencing, phone calls, and/or face to face visits. Reporting will be focused on the achievement of stated milestones as indicated in the project timeline. The OAC reserves the right to request additional updates, site visits, or reporting.
Links and References to Relevant AHA Policies
- Public Access: The AHA’s public access policy requires that all journal articles resulting from AHA funding be made freely available in PubMed Central (PMC) and attributed to a specific AHA award within 12 months of publication. It is the responsibility of the awardee to ensure journal articles are deposited into PMC.
- Open Data: Any factual data that is needed for independent verification of research results must be made freely and publicly available in an AHA-approved repository as soon as possible, and no later than the time of an associated publication or the end of the award period (and any no-cost extension), whichever comes first. For more information on the above policies, see AHA's Open Science Policy webpage.
- Preregistration: AHA requires preregistration for any funded clinical trials and encourages preregistration for any studies that make an inferential claim from a sampled group or population, as well as studies that are reporting and testing hypotheses. After a project is completed, protocols and preregistration analysis plans can be used in conjunction with the final study and analysis by researchers seeking to replicate, reproduce, and build upon findings. See AHA’s preregistration information.
- Other: The projects described can have no scientific or budgetary overlap with other funded work. Any inventions, intellectual property, and patents resulting from this funding are governed by the AHA Intellectual Property Policy for Research Funding EXCEPT to the extent modified by specific Intellectual Property terms for this award mechanism, including financial terms, which will be communicated to awardees following the review process. The applicant/awardee and institution are responsible for compliance with all AHA research award policies and guidelines for the duration of any awards they may receive. Visit the Research Programs Awards Policies page for more information on this topic: AHA Policies Governing All Research Awards.
References
- CDC Ntl Ctr for Health Statistics, Heart Disease Deaths 2023, https://www.cdc.gov/nchs/hus/topics/heart-disease-deaths.htm
- Mehta et al., Circulation 133: 916-947, 2016
- Gao et al., Med Novel Tech Devices 4 https://doi.org/10.1016/j.medntd.2019. 00025, 2019
- Peters, S.A.E. and Woodward, M, Curr Diabetes Rep 18:33 https://doi.org/10.1007/s11892-018-1005-5, 2018
- O’Kelly et al., Circ Res 130: 652-672, 2022
- Agarwala et al., Circulation 141: 592-599, 2020
- Agarwala et al., Curr Cardiovasc Risk Rep 2019; 13(7): doi:10.1007/s12170-019-0616-y.
- Rajendran et al., Atherosclerosis 2023; 384: https://doi.org/10.1016/j.atherosclerosis 2023, 117269
- Solomon DH, et al. Ann Rheum Dis 2023;82:324–330. doi:10.1136/ard-2022-223302
- Ndumele et al., Circulation 148: 1606-1635, 2023
- Ndumele et al., Circulation 148: 1636-1664, 2023
- Aggarwal, et al., JAMA 331: 1858-1860, 2024
- Tsao, et al., Circulation 147: e93-e621, 2023
- Yoshida et al., JAMA Network Open. 2022;5(7): e2222070. doi:10.1001/jamanetworkopen.2022.22070
- Chronic Kidney Disease in the United States, 2023, https://www.cdc.gov/kidney-disease/php/data-research/?CDC_AAref_Val=https://www.cdc.gov/kidneydisease/publications-resources/CKD-national-facts.html
Specialties
Eligibility Requirements
Required Pre-proposal
Each Center Director is required to submit a pre-proposal electronically via ProposalCentral.
How to Submit a Pre-Proposal in ProposalCentral (PDF)
Applicant institutions MUST be AREA eligible (as defined by the NIH) or partner with an AREA eligible institution. As part of the required Pre-proposal, applicants must upload a letter from a Senior Institutional Official (e.g., president, provost, dean, etc.) from the AREA eligible institution affirming their eligibility.
AHA staff will review for compliance. A non-complying institution will not be permitted to submit a full proposal. This administrative review is part of the Pre-proposal process, which is required and, though rare, may prevent an applicant from moving forward. Even though the Pre-proposal is required, each Center and Project applicant is advised to begin planning and designing their applications before the Pre-proposal deadline to maximize the amount of time available to develop their full proposal.
SUBMISSION
Pre-Proposals must be submitted electronically via ProposalCentral. Applicants can create required documents in advance; refer to the required application documents. All submissions require the signature of a designated institutional representative.
Eligible Countries:
Sponsor Details
Sponsor Institute/Organizations: American Heart Association
Sponsor Type:
Address: National Center 7272 Greenville Ave. Dallas, TX 75231 Customer Service 1-800-AHA-USA-1 1-800-242-8721
Legal & Affiliation Disclaimer
Affiliation Disclaimer: Trialect operates independently and is not affiliated with, endorsed by, or supported by any sponsors or organizations posting on the GrantsBoard platform. As an independent aggregator of publicly available funding opportunities, Trialect provides equal access to information for all users without endorsing any specific funding source, content, organization, or sponsor. Trialect assumes no responsibility for the content posted by sponsors or third parties.
Subscription Disclaimer: Upon logging into Trialect, you may choose to SUBSCRIBE to GrantsBoard for timely notifications of funding opportunities and to access exclusive benefits, such as priority alerts, reminders, personalized recommendations, and additional application support. However, users are advised to contact sponsors directly for any questions and are not required to subscribe to engage with funding opportunities.
Content Ownership and Copyright Disclaimer: Trialect respects the intellectual property rights of all organizations and individuals. All content posted on GrantsBoard is provided solely for informational purposes and remains the property of the original owners. Trialect does not claim ownership of, nor does it have any proprietary interest in, content provided by third-party sponsors. Users are encouraged to verify content and ownership directly with the posting sponsor.
Fair Use Disclaimer: The information and content available on GrantsBoard are compiled from publicly accessible sources in alignment with fair use principles under U.S. copyright law. Trialect serves as an aggregator of this content, offering it to users in good faith and with the understanding that it is available for public dissemination. Any organization or individual who believes their intellectual property rights have been violated is encouraged to contact us for prompt resolution.
Third-Party Posting Responsibility Disclaimer: Trialect is a neutral platform that allows third-party sponsors to post funding opportunities for informational purposes only. Sponsors are solely responsible for ensuring that their postings comply with copyright, trademark, and other intellectual property laws. Trialect assumes no liability for any copyright or intellectual property infringements in third-party content and will take appropriate action to address any substantiated claims.
Accuracy and Verification Disclaimer: Trialect makes no warranties regarding the accuracy, completeness, or reliability of the information provided by sponsors. Users are advised to verify the details of any funding opportunity directly with the sponsor before taking action. Trialect cannot be held liable for any discrepancies, omissions, or inaccuracies in third-party postings.
Notice and Takedown Policy: Trialect is committed to upholding copyright law and protecting the rights of intellectual property owners. If you believe that content on GrantsBoard infringes your copyright or intellectual property rights, please contact us with detailed information about the claim. Upon receipt of a valid notice, Trialect will promptly investigate and, where appropriate, remove or disable access to the infringing content.
Grant
Letter Of Intent Deadline:
Oct 30, 2024
Final Deadline:
Jan 22, 2025
Funding Amount:
$4,400,000
3 awards available.
Host Details
Affiliation: American Heart Association
Address: National Center 7272 Greenville Ave. Dallas, TX 75231 Customer Service 1-800-AHA-USA-1 1-800-242-8721
Website URL: https://professional.heart.org/en/research-programs/aha-funding-opportunities/sfrn-on-ckms-heterogeneity-in-women
Disclaimer:It is mandatory that all applicants carry workplace liability insurance, e.g., https://www.protrip-world-liability.com (Erasmus students use this package and typically costs around 5 € per month - please check) in addition to health insurance when you join any of the onsite Trialect partnered fellowships.






















